Built to lurk: the regulatory circuit behind stomach bugs
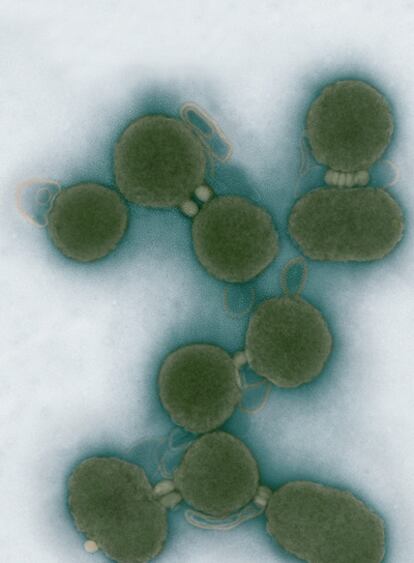
In the same period, pathologist Robin Warren and physician Barry Marshall were examining people affected by gastritis- inflammation of the stomach lining - and repeatedly discovered distinctive spiral-shaped bacteria associated with the gastric lesions in their bellies. In 1982 they managed to culture the bugs and Marshall bravely drank a flask full of them, hypothesising that he would develop gastritis soon after. Indeed, several days later he started to develop acute symptoms, and the same type of helical bacteria was found in his stomach. Warren and Marshall also showed that antibiotics could be used to cure the infection. The bacterial species they had discovered was named Helicobacter pylori.
Nowadays if you ask a physician for remedies against your gastritis, he or she will check for the presence of H. pylori and prescribe suitable antibiotics. However, some strains of this bacterium are becoming resistant to antibiotic treatment. Untreated infections cause acute and chronic stomach diseases, which develop into gastric cancer in 1% of infected people. Moreover, the bacteria can lurk in the stomach without causing any symptoms for up to decades, and then suddenly become pathogenic. In doing so, they express a set of genes that code for molecules and virulence factors, which enable persistent infections.
This process of gene expression is highly controlled. Infecting bacteria need to tightly regulate the production of virulence factors and, like an invading army, act tactically. Certain compounds required for battle only need to be synthesised in specific environments (for example, in response to stomach acidity in the case of H. pylori). It would be inefficient to express the associated genes constantly in the absence of a real need: this would drain precious resources and make the bacterial army poorly equipped to deal with a counter-offensive by the well-organised human immune system.
Accordingly, the expression of genes can be finely tuned through a set of regulator molecules, which are able to 'sense' a particular stimulus and activate, block or modulate the amount of virulence factor produced from a given gene.
In the cell, these biological switches rarely work as stand-alone devices. Many are wired in a network, like an electrical circuit, that confers specific dynamics to the regulatory response. This circuit (called the transcriptional regulatory network, TRN) represents a sort of motherboard that controls the decision making of the bacterium: whether to move or to arrest, to divide or to vegetate, to attack or to hide. The regulators in the TRN act like a panel of generals and colonels to determine which bacterial genes are expressed and, therefore, which systems will be executed to make the bacterium infectious.
Most pathogens possess hundreds of specialised regulators, each dedicated to a specific task, such as controlling the expression of a virulence factor. H. pylori, however, possesses only very few regulators. So how are the complex responses needed to infect humans regulated in this pathogen? If few commanders are present, who is making the decisions?
At the University of Bologna, we have studied single regulators as well as their wiring and have found that H. pylori has a very peculiar regulatory circuit. Rather than having a plethora of specialised regulators, the TRN uses different combinations of small sets of regulators to infect the host. In other words, the TRN employs units of a few multitasking switches that work closely together - like highly trained SWAT teams - rather than lots of isolated stand-alone commanders.
Our research suggests that the sudden activation of virulence is probably not caused by a single, programmed switch but is the result of balanced action by a combination of regulators, which explains why H. pylori is so efficient at maintaining persistent, life-long infections. Understanding the mechanisms that underlie this balance may help us pin point key pathways in the bacterium that we can target with drugs in order to counter infection and prevent gastric cancer.
Clearly, the activity of regulators in the TRN of pathogenic bacteria can have a tremendous impact on our lives and deserve systematic investigation. H. pylori is a good place to start because the TRN is not wired up to large a number of regulators. Discoveries in this model system could subsequently be used to help dissect the TRN of other pathogenic bacteria. In the long term, such research will be useful for development of next generation therapeutic agents and medical applications that can eradicate antibiotic resistant H. pylori and other important human pathogens. Or is anybody eager to give up a tasty life?
Alberto Danielli, University of Bologna. www.atomiumculture.eu
Tu suscripci¨®n se est¨¢ usando en otro dispositivo
?Quieres a?adir otro usuario a tu suscripci¨®n?
Si contin¨²as leyendo en este dispositivo, no se podr¨¢ leer en el otro.
FlechaTu suscripci¨®n se est¨¢ usando en otro dispositivo y solo puedes acceder a EL PA?S desde un dispositivo a la vez.
Si quieres compartir tu cuenta, cambia tu suscripci¨®n a la modalidad Premium, as¨ª podr¨¢s a?adir otro usuario. Cada uno acceder¨¢ con su propia cuenta de email, lo que os permitir¨¢ personalizar vuestra experiencia en EL PA?S.
?Tienes una suscripci¨®n de empresa? Accede aqu¨ª para contratar m¨¢s cuentas.
En el caso de no saber qui¨¦n est¨¢ usando tu cuenta, te recomendamos cambiar tu contrase?a aqu¨ª.
Si decides continuar compartiendo tu cuenta, este mensaje se mostrar¨¢ en tu dispositivo y en el de la otra persona que est¨¢ usando tu cuenta de forma indefinida, afectando a tu experiencia de lectura. Puedes consultar aqu¨ª los t¨¦rminos y condiciones de la suscripci¨®n digital.




























































